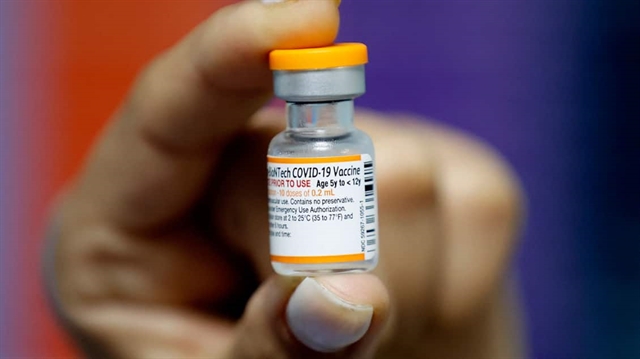
HÀ NỘI — The Vietnamese Government has approved the Ministry of Health’s proposal to purchase 21.9 million doses of Pfizer/BioNTech COVID-19 vaccines for children from five to under 12 years old.
The contractor selection will follow the special circumstances model specified in Article 26 of the Law on Bidding, due to the urgent nature of ongoing pandemic prevention and control efforts.
The Ministry of Health is to be responsible for organising the purchase and deployment of Pfizer’s COVID-19 vaccine for children from 5 to under 12 years old, ensuring safety and effectiveness.
A survey from the ministry indicated that about 50 per cent of parents in the country agree to let their children in the 5-11 age group get the vaccines, with further details on this survey expected after the Tết (Lunar New Year) holiday that ended on Sunday.
There is also no information yet available on the rollout plan of the vaccines, but it has been suggested that children in this age group will only be recommended to get the shots, no mandate will be enforced.
Health minister Nguyễn Thanh Long in an interview with local media last month has admitted administration to this age group would be more difficult than the rollout for adults and teenagers aged 12-17 that the country has been doing, and it has accepted the likelihood that there will be some amount of doses going unused.
181.1 million doses of COVID-19 vaccines have been administered in Việt Nam with about 98 million people, including 165.4 million in adults – 70.6 million first doses, 67.8 million second doses, 10.3 million additional doses and 16.6 million booster doses.
16.25 million doses of Pfizer vaccines have been given to children aged 12-17 years, including 7.8 million second doses.
Pfizer shots so far are the only ones to have been recommended by the World Health Organisation (WHO) for use in children this young, with a lower dosage compared to the adult shots.
“WHO recommends that countries should consider using the vaccine in children aged 5 to 17 only when high vaccine coverage with two doses has been achieved in the high priority groups as identified in the WHO Prioritization Roadmap,” WHO said in a statement.
“Children and adolescents aged 5-17 years of age with comorbidities that put them at significantly higher risk of serious COVID-19 disease, should be offered vaccination, alongside other high-risk groups.” — VnExpress News
- Reduce Hair Loss with PURA D’OR Gold Label Shampoo
- Castor Oil Has Made a “Huge” Difference With Hair and Brow Growth
- Excessive hair loss in men: Signs of illness that cannot be subjective
- Dịch Vụ SEO Website ở Los Angeles, CA: đưa trang web doanh nghiệp bạn lên top Google
- Nails Salon Sierra Madre
 VnExpress News The News Gateway of Vietnam
VnExpress News The News Gateway of Vietnam