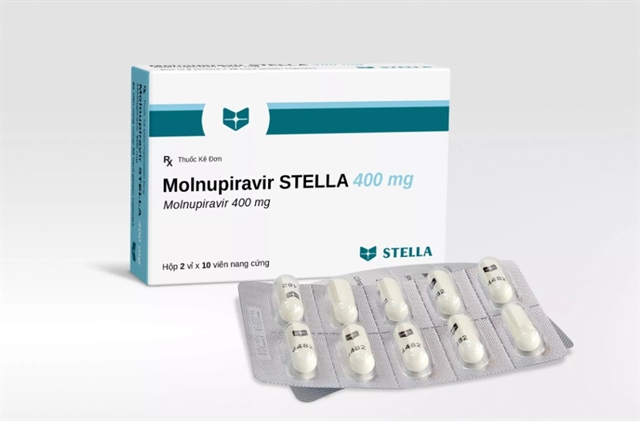
HÀ NỘI — The Drug Administration of Việt Nam under the Ministry of Health has issued the list of three COVID-19 treatment drugs produced in Việt Nam that contain the antiviral ingredient Molnupiravir, which have been granted certificates of registration for conditional use.
The three products – Molravir 400mg produced by Boston Việt Nam Pharma (based in Bình Dương Province), Movinavir 200mg manufactured by Mekophar Chemical Pharmaceutical (based in HCM City), and Molnupiravir Stella 400mg produced by Stellapharm J.V Co., Ltd (Bình Dương Province) – were the first to be approved for use in Việt Nam.
The products are hard gelatine capsules, with an expiration date of six months.
Each course comprises 20 tablets of 400mg, for a price of about VNĐ300,000 (US$13) as recommended by the health ministry.
Earlier, the advisory council of the Ministry of Health agreed with the proposal on granting certificates of registration for conditional circulation to the three drugs. The licensing for these drugs is valid for three years from the date of signing the decision.
The drug is prescription-only and used to treat mild to moderate adult COVID-19 patients with at least one risk factor that could enhance progression of coronavirus.
The recommended dosage is 800mg for every 12 hours over five days, and the medicines should be taken as soon as possible after a positive COVID-19 diagnosis and within five days of the symptoms appearing.
In the caution section, it is noted that the drug ‘must not be used as pre-exposure and post-exposure prevention.’
The drug must not be used for people under 18 years as it might affect the development of bones and cartilages, or pregnant women for it could result in complications for both mothers and the foetuses – including serious birth defects and miscarriages.
It’s also noted that the data on the impacts of the drug on sperm have not been available with studies still underway.
The DAV requested drug manufacturing and registration establishments to produce in accordance with dossiers and documents registered with the Ministry of Health, coordinate with treatment facilities to strictly comply with current regulations on prescription drugs, monitor the safety, effectiveness, and unwanted effects of drugs on Vietnamese people, and report regularly.
The DAV also asked the Departments of Health of provinces and centrally-run cities to direct local medical examination and treatment facilities, medical staff, and drug supply establishments to notify patients of the benefits and risks when using these drugs, treatment methods, other drugs that can replace Molnupiravir in treating COVID-19.
During the circulation of the three drugs, based on the monitoring and updating information on the safety and effectiveness of them, the agency may decide to revoke the granted certificates of registration for circulation in line with Clause 1, Article 58 of the Law on Pharmacy. — VnExpress News
- Reduce Hair Loss with PURA D’OR Gold Label Shampoo
- Castor Oil Has Made a “Huge” Difference With Hair and Brow Growth
- Excessive hair loss in men: Signs of illness that cannot be subjective
- Dịch Vụ SEO Website ở Los Angeles, CA: đưa trang web doanh nghiệp bạn lên top Google
- Nails Salon Sierra Madre
 VnExpress News The News Gateway of Vietnam
VnExpress News The News Gateway of Vietnam