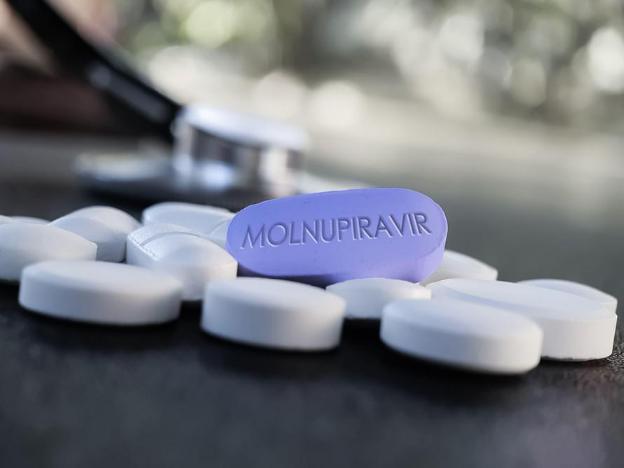
HÀ NỘI — The COVID-19 drug for patients with mild symptoms – Molnupiravir – must only be used with a doctor’s prescription following the indications, limits of use and warnings of the drug, doctors have reaffirmed after safety concerns were reported.
Doctors and health authorities on Tuesday called on people not to buy or use the drug Molnupiravir without doctors’ advice, or use the drug with unknown origin.
If encountering any adverse drug reactions when using Molnupiravir, people should immediately notify a doctor or pharmacist for advice and treatment.
The call was made after foreign and local media reported safety concerns regarding the drug. Some publications reported that the India Council of Medical Research (ICMR) has decided not to include the antiviral drug Molnupiravir in its Clinical Management Protocol.
In Việt Nam, the drug was first used for COVID-19 patients with mild sickness in HCM City last August.
As of January 8, 2022, the Health Ministry had allocated 53 localities more than 400,000 doses of the drug to treat COVID patients with mild sickness.
The Molnupiravir Controlled Treatment Pilot Program for mild cases of COVID adheres to a rigorous scientific research protocol that has been approved by the National Ethics Committee for Biomedical Research and the Ministry of Health.
At a meeting on January 5, the Advisory Council for issuance of circulation certificates of drugs and medicinal ingredients agreed to propose the Ministry of Health grant conditional circulation registration certificates for three drugs containing the active ingredient Molnupiravir.
The UK Medicines and Healthcare Products Regulatory Agency on December 4, 2021, approved Molunupiravir under special conditions for treatment of mild to moderate COVID-19 in adults who have at least one risk factor for developing severe illness.
US Food and Drug Administration (FDA) on December 23 granted Emergency Use Authorisation for the drug for treating mild-to-moderate COVID-19 in adults at high risk for progression to severe disease. Japan and a number of other countries also approved the drug’s use.
Special attention should be paid when using Molnupiravir to treat COVID-19: Molnupiravir is used to treat mild to moderate COVID-19 in adults who are positive for a diagnostic test for SARS-CoV-2 and have at least one risk factor for severe disease progression.
The drug is only to be used in patients when the symptom onset is less than five days. Molnupiravir must not be used for more than five consecutive days. Molnupiravir is not used for post- or pre-exposure prophylaxis against COVID-19.
Molnupiravir is not recommended for use during pregnancy. Women of childbearing potential should use effective contraception during treatment and for four days after the last dose of Molnupiravir.
Based on the potential for adverse reactions in the neonate in Molnupiravir, breastfeeding is not recommended during treatment and for four days after the last dose of Molnupiravir.
Molnupiravir is not authorised for use in patients under 18 years of age because of possible effects on bone and cartilage development.
Molnupiravir can affect sperm although the risk is low. Therefore, men who are sexually active with women of childbearing potential should use a reliable method of contraception during treatment and for at least three months after the last dose of Molnupiravir.
On Tuesday, the Health Ministry also announced that the health authority is waiting for an official announcement from the Indian counterpart about not including Molnupiravir in treating COVID-19. — VnExpress News
- Reduce Hair Loss with PURA D’OR Gold Label Shampoo
- Castor Oil Has Made a “Huge” Difference With Hair and Brow Growth
- Excessive hair loss in men: Signs of illness that cannot be subjective
- Dịch Vụ SEO Website ở Los Angeles, CA: đưa trang web doanh nghiệp bạn lên top Google
- Nails Salon Sierra Madre
 VnExpress News The News Gateway of Vietnam
VnExpress News The News Gateway of Vietnam